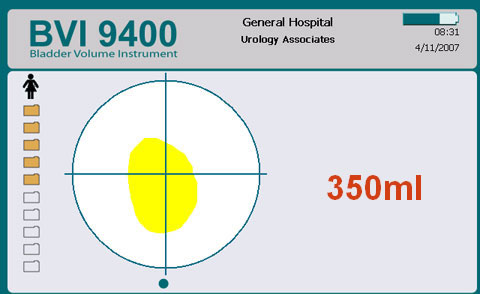
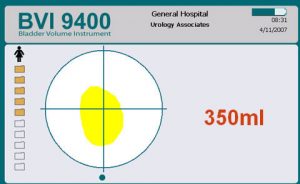
Following assessment and post void Bladder Scan you establish that there is a residual volume of approx. 350mls of urine in his bladder.
Q. Which of the following assessment and treatment options may be appropriate at this time?
See VTA for explanation of Appropriate assessment and treatment options.

Verathon Medical UK This is an example of a bladder scanner. Other models are available.
- Non-invasive portable cone of ultrasound, frequently used to estimate the residual volume of urine in the bladder.
- Bladder scanning is an alternative to in/out catheter.
- Is able to differentiate between fluid and tissue
- Battery operated
List of assessment options:
Q. What should be your 3 priorities from this list? Drag 3 of the following options.
Once completed the task, click on the View text alternative button to read more.
Scenario

Mr Robert Smith is a 73 year old gentleman. He is admitted to your ward with a profound right sided weakness. He is immobile and requires a hoist for all transfers. He has expressive dysphasia and on admission appears very confused and agitated.
He presents not having passed a good volume of urine for approx 48 hrs, he appears to continuously dribble small amounts of urine. Robert has had two episodes of faecal incontinence passing very loose foul smelling stool, it is unclear when he last had a normal formed bowel motion.
Medical History: Type 2 diabetic, Hypertension.
Medications: Fybogel™, furosemide, nifedipine, metformin and amitriptyline (for more information on each of these medications see “Additional Information”).
- Use of containment products does not actively support recovery of bladder/bowel function.
- Containment products should only be used as a last resort when all other bladder/bowel rehabilitation has failed.
Assessing for a product is not a bladder/bowel assessment. It is only a part of a wider comprehensive assessment. Products can improve a patient’s quality of life, protect dignity and encourage functional and social independence when recovery of bladder/bowel function is not possible.
The main types of containment are:
-
- Absorbent pads
- Sheath catheters
- Indwelling urethral/suprapubic catheters only where clinically indicated (RCP 2016, Australian Stroke Foundation 2017).
Factors to be considered for choice of containment:
- Level of disability: some products are easier to use than others
- Physical and cognitive function
- Type and severity of incontinence
- Gender
- Skin integrity
- Availability of care
- Likely duration of use
It is vital that before supplying a patient with a containment product, clear instructions about the correct use, fitting, storage and disposal of the product are given.
Most common side effects include:
- Dry mouth (Most common)
- Dry eyes
- Blurred vision
- Constipation
- Dizziness
- GI disturbances e.g. Nausea, Dyspepsia, Abdominal pain
- UTI / cystitis
- Insomnia
Specific side-effects of mirabegron include:
- UTI,
- nausea
- cardiac problems – tachycardia / palpitations
NB: Prescriber should be aware of contra indications and note that Antimuscarinic drugs should be used with caution in older people (especially if frail).
Antimuscarinic medication for an overactive bladder:
- Vesicare (Solifenacin) 5mg / 10mg once daily
- Detrusitol XL (Tolterodine) 4mg once daily
- Lyrinel XL (Oxybutinin) 5mg / 10mg once daily
- Kentera Patch (Oxybutinin transdermal) applied every 3-4 days
- Toviaz (Fesoterodine) 4mg and 8mg once daily
- Regurin (Trospium chloride) 20mg BD
- Emselix (Darifenicin) 7.4 / 15mg once daily
Beta-3 adrenergic receptor agonist medication:
- Mirabegron (Betmiga) 25mg / 50mg once daily
- Antimuscarinic agents and beta3-receptor agonists act to relax the detrusor muscle of the bladder wall.
- Most patients will report a reduction to their symptoms within the first 2 to 4 weeks of use.
- The need for continuing drug therapy should be reviewed after 3 to 6 months. If taken long term, they should be reviewed yearly.
- Must not be given when person has incomplete emptying or urinary retention as it may make this worse.
- Consult your local formulary for choice of drug.







